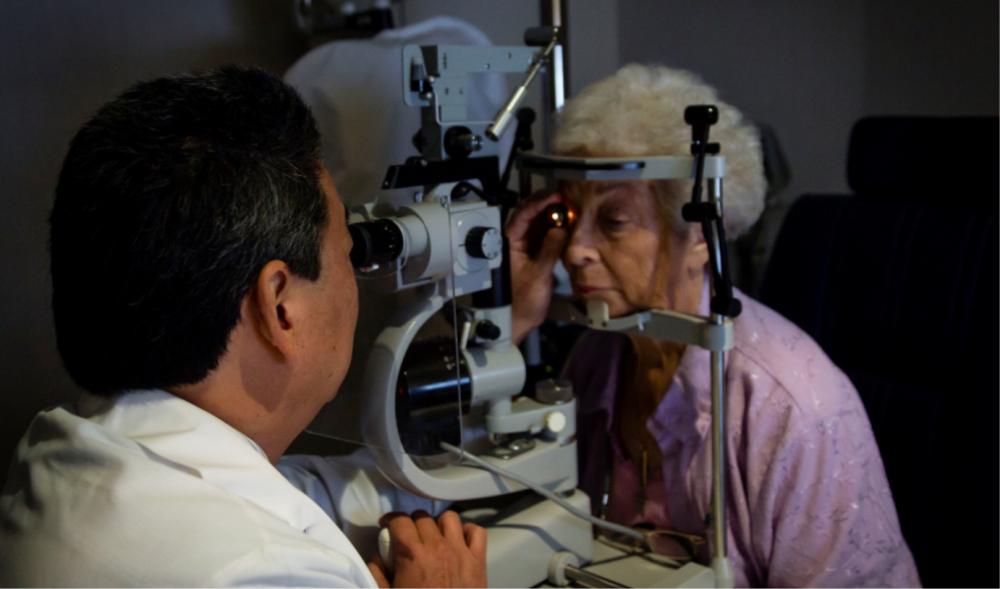
Hawaii Macula and Retina Institute (HMRI) was established in 1993 as a center of excellence for the study of retinal diseases. HMRI is an affiliate of The Retina Center at Pali Momi and Retina Consultants of Hawaii. The Retina Center at Pali Momi is a hospital-based research program affiliated with Hawaii Pacific Health. This center has allowed retinal research to be a center of excellence for the Pali Momi Medical Center and allowed our research program to make use of hospital resources, the Pali Momi foundation and continuing medical education programs. Dr. Gregg Kokame was the initial founder and remains the medical director of the research and educational programs. This academic program has brought world renowned leaders in the retinal field to Hawaii to speak. The research program of HMRI has developed worldwide recognition with presentations at major meetings around the world and publications in the leading research journals in ophthalmology. HMRI consistently participates in the latest multi-centered national and international clinical trials for the development of new treatments for retinal diseases such as macular degeneration, diabetic retinopathy, and retinal vein occlusions.
Dr. Sarah Read is the assistant medical director of HMRI. Both she and Dr. Kokame trained at the renowned Bascom Palmer Eye Institute, which has been rated the number one eye institute for the past 20 years. The other doctors supporting HMRI are Dr. James Lai and Dr. Raymond Wee.
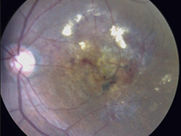
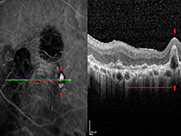
All doctors have taken on the role of Principal Investigator for clinical trials. HMRI has now participated in over 60 multi-centered clinical trials. Clinical trials run at HMRI have led to FDA approval by the FDA of revolutionary new treatments for retinal diseases, including the most revolutionary treatment for exudative macular degeneration, diabetic retinopathy, and blood vessel occlusions in the retina. The very first injection of the new treatment for exudative macular degeneration was performed at HMRI in 1993. These injectable medications have become the standard of care for retinal diseases. HMRI has been the only clinical center in Hawaii leading to FDA approval of Lucentis, Eylea, Beovu, and Faricimab. We currently are participating in clinical trials of promising new medications that last longer than our current treatments Dr. Kokame has also designed and run unique clinical trials studying treatment options for polypoidal choroidal vasculopathy (PCV), a subtype of exudative macular degeneration, which has created new knowledge in better understanding this disease and how to manage this disease. PCV is underdiagnosed in the United States, due to lack of access to and lack of education in indocyanine green angiography. More recently, research done at HMRI has shown that PCV is less responsive to our usual medications for exudative macular degeneration, and has unique characteristics in different ethnicities. PCV research here at HMRI has led to presentations at numerous national meetings such as the American Academy of Ophthalmology, Macula Society, Retina Society, and American Society of Retina Specialists. Dr. Kokame has been an invited speaker at some of the most prestigious meetings such as American Ophthalmological Society, Aspen Retinal Detachment Society, Pan-American Association of Ophthalmology, World Ophthalmology Congress, Asia Pacific Vitreoretinal Society, and many other invited lectures all over the world, including the Pettit Lecture at the Jules Stein Eye Institute in 2014. Dr. Kokame has become a world recognized leader in PCV and doctors around the world consult him on PCV management.
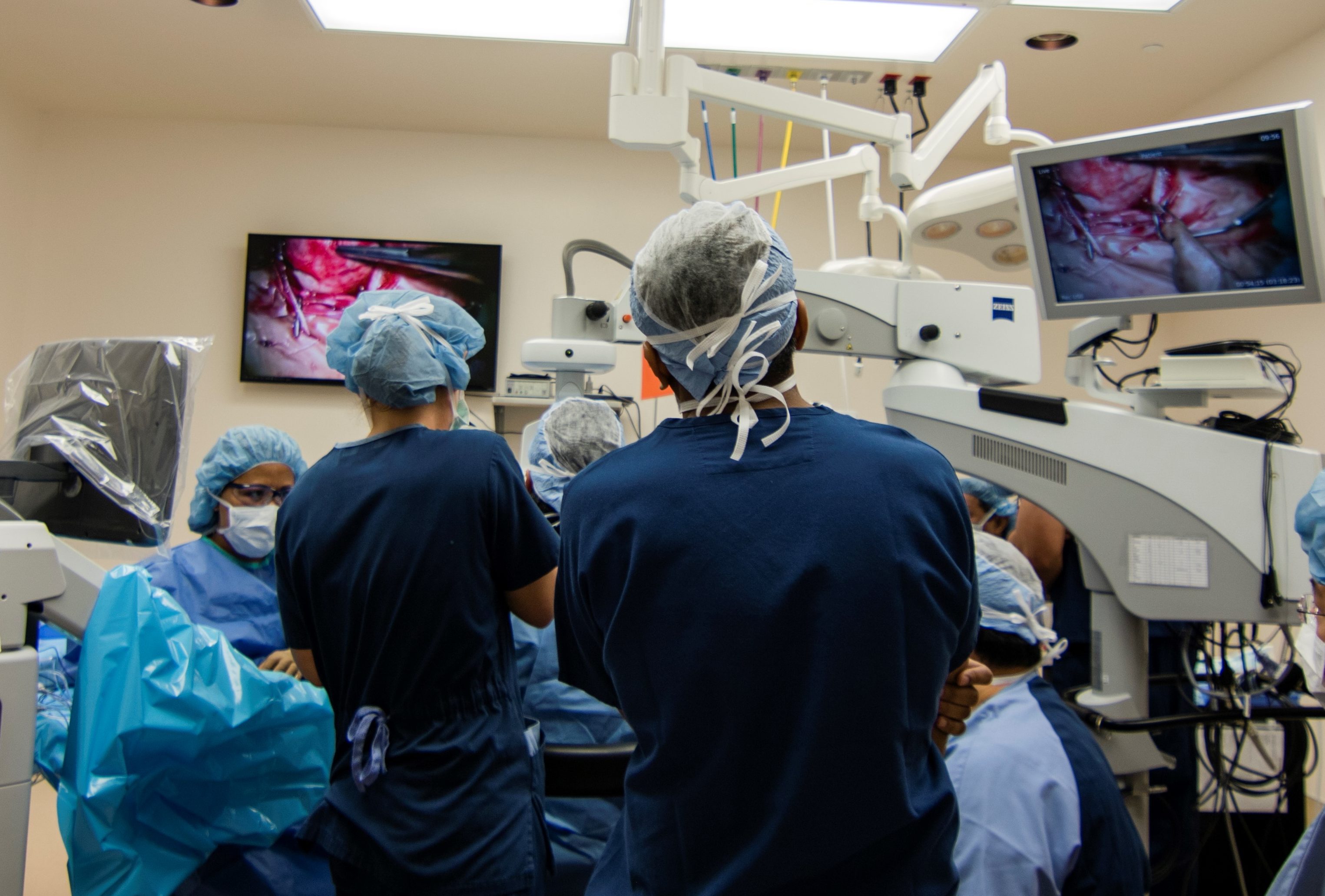
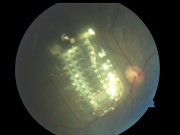
HMRI has been pioneering new surgical techniques to help improve retinal surgeries for both the patient and surgeon. These range from scleral fixation of dislocated posterior chamber intraocular lenses, and various management options for macular holes. Because of this surgical expertise, HMRI was the first site in the Asia-Pacific region to perform the “Bionic Eye” surgery for retinitis pigmentosa, which is a disease which can result in complete blindness due to loss of the photoreceptors.
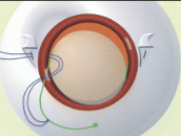
More recently, Dr. Sarah Read was the first to perform surgery to implant a new reservoir called the port delivery system (PDS) that allows slow release of Lucentis, which will help with the biggest unmet need for treatment of retinal diseases – decreasing the number of injections required.
One of the most important new frontiers in medicine is the use of gene therapy. This can treat not only uncommon hereditary retinal diseases, but also very common sight-threatening disease. Dr. Gregg Kokame performed the first gene therapy procedure for exudative macular degeneration, which is a first for Hawaii not only in ophthalmology but in all of medicine in Hawaii. Gene therapy involves the use of a vector, which is a modified virus without the viral genetic material, but allows transport of genetic material into the DNA of cells. RGX-314 is a gene encoding for Lucentis for exudative macular degeneration in this trial. Surgery is required to place the gene therapy under the retina. RGX-314 is designed not to alter human DNA, not to cause significant inflammation, and not to cause other illnesses in humans. Gene therapy will be important for all areas of medicine in the future. But the era of gene therapy in Hawaii has already started with this amazing advance in the management of retinal diseases. This treatment is again important in decreasing the number of injections, and may even allow some patients to no longer require injections. As a result of research and clinical explorations, HMRI has authored over 100 peer-reviewed publications to major ophthalmology journals as well as book chapters. The hope is that through these publications, HMRI will help to advance the field of retinal disease management and surgery by its unique contributions.
Retinal Research with Worldwide Impact!
Some of HMRI’s major accomplishments:
- Help a blind patient with retinitis pigmentosa see again
- Cure a disease that was untreatable for over 100 years
- Advance vitreoretinal surgery to diseases which previously were not approachable with surgery
- Contribute to the greatest advancement in retinal treatments for the one of the leading causes of vision loss, exudative macular degeneration
- Pioneer new surgical techniques in the management of complications of the most common surgical procedure performed in the United States, cataract surgery.
- Helped to unlock the secrets of a newly recognized cause of leaking and bleeding in the central vision area of the eye (macula) that is much more common in patients of Asian descent – Polypoidal choroidal vasculopathy
- Create awareness of new, key information on the disease polypoidal choroidal vasculopathy
- Beginning the gene therapy era of treatment in Hawaii
- Surgery to place an implant that allows slow release of medications
Click here for more stories on HMRI’s latest research and successes!