Macular degeneration is the most common cause of vision loss in Hawaii and in the United States. This degeneration or scarring involves central vision or the macula, and results in loss of reading vision, driving vision and facial recognition. The dry type of macular degeneration tends to progress slowly and has a more gradual course but puts the eye at risk for exudative macular degeneration.
Exudative macular degeneration (wet type), the subtype most commonly causing vision loss, has been successfully treated by intravitreal injections or injections into the eye, which are performed comfortably in the office with topical anesthesia. However, many cases require multiple injections even monthly, and thus are a significant treatment burden on both the patient and the doctor. These injections involve anti-vascular endothelial growth factor (anti-VEGF) medications, which inhibit abnormal blood vessel growth and leakage and bleeding from abnormal blood vessels.
Regnexbio is a gene therapy company which has targeted exudative macular degeneration by use of RGX-315, which is a precisely coded vector that allows the eye to produce its own anti-VEGF medication. This involves the use of an adeno-associated virus8 (AAV8), which transfers the gene into the cells and allows the cells to produce an-anti-VEGF protein, very similar to Lucentis. Lucentis was the first anti-VEGF medication approved by the FDA in October of 2006. Dr. Kokame was the principal investigator of the Hawaii clinical study site for the Lucentis trials which led to FDA approval and which were performed at the Hawaii Macula and Retina Insititute. With gene therapy the eye becomes its own factory for making Lucentis for the treatment of exudative macular degeneration. AAV8 is not known to cause human disease, has most of the viral elements removed to allow transfer of the specific gene, and does not alter the human genome.
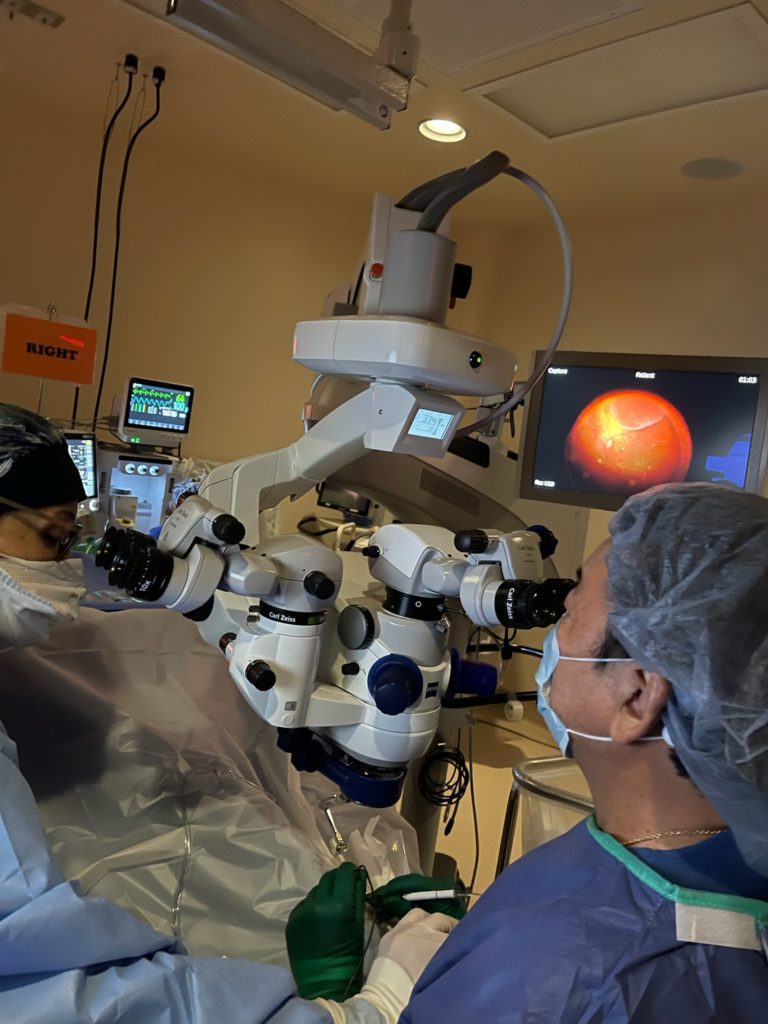
This procedure is performed during eye surgery which removes the central vitreous and allows the RGX-315 to be placed into the subretinal space (under the retina).
This treatment for exudative macular degeneration will be the first human gene therapy treatment in Hawaii. It will be performed by Dr. Gregg Kokame on 11/24/21 at the Eye Surgery Center of Hawaii in the Atmosphere trial sponsored by Regenxbio. Dr. Gregg Kokame is the principal investigator for the Atmosphere trial in Hawaii.
Dr. Kokame at the Eye Surgery Center of Hawaii was also the first to perform the bionic eye surgery in the Asia Pacific region March 24, 2015.
Dr. Gregg T Kokame is Chief of Ophthalmology and Clinical Professor at the University of Hawaii John A Burns School of Medicine, medical director of the Hawaii Macula and Retina Institute, and founding partner and senior consultant at Retina Consultants of Hawaii. Dr. Gregg Kokame is President of the Eye Surgery Center of Hawaii.
The Eye Surgery Center of Hawaii is the largest operating center for eye disease in Hawaii and includes eye surgeons in multiple specialties within ophthalmology. There are 4 operating rooms, and a skilled and compassionate staff highly trained specifically for all of the subspecialties of eye surgery, including cataract surgery, glaucoma surgery, corneal surgery, oculoplastic surgery and vitreoretinal surgery. The Eye Surgery Center of Hawaii has been on the leading edge of the study of many innovative surgical treatments in ophthalmology.

Comments are closed.