What is Polypoidal Choroidal Vasculopathy (PCV)? What are the current methods of diagnosing and treating PCV?
Read an interview with Dr. Kokame, Dr. Koh and Dr. Spaide discussing PCV in the American Academy of Ophthalmology Retina December 2012 Clinical Update on Polypoidal Choroidal Vasculopathy.
It’s a question that has been controversial in ophthalmology over many years: Is polypoidal choroidal vasculopathy (PCV) a subtype of neovascular age-related macular degeneration (AMD) or a separate clinical entity? Even more important, does this distinction affect treatment and outcomes?
Genetic studies suggest that PCV is a type of choroidal neovascularization (CNV), and other research has demonstrated that the anti-VEGF therapies used for AMD may improve vision in patients with PCV. Yet, in contrast to AMD, some PCV patients fail to respond to anti-VEGF treatment and do better with verteporfin photodynamic therapy (PDT). Recent studies indicate that the combination of PDT and an anti-VEGF agent provides added benefit in treating PCV.1-3
How can clinicians apply these findings to their own patients? Three vitreoretinal specialists discuss the implications of recent research and their approach to managing this puzzling condition.
Comparing the Conditions
Similarities. While some researchers believe that PCV and neovascular AMD are different diseases, the conditions share some fundamental similarities. Among these are abnormal growth of new blood vessels and fluid accumulation under the retinal pigment epithelium as well as sequelae that include subretinal hemorrhage and pigment epithelial detachment (PED). In both conditions, loss of vision results from bleeding, leakage, and scar tissue formation, according to Richard F. Spaide, MD, of Vitreous Retina Macula Consultants in New York. Dr. Spaide was one of the authors of the first paper describing PCV, published in 1990.4
“Although some published research papers have said that PCV is a different clinical entity than AMD, there’s really very little proof that these are different diseases when you look at the totality of the scientific evidence,” he said.
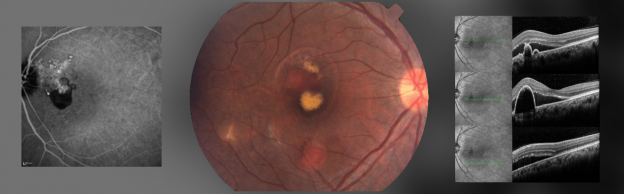
Contrasting characteristics. Nevertheless, PCV has distinctive clinical features. For example, it presents as unilateral disease more often than does AMD. Another important sign of PCV, said Dr. Spaide, is a branching vascular network (BVN) with interconnected reddish-orange dilated vessels, especially when it occurs in a younger patient or in one with long-standing disease. Clinically apparent orange-red nodular structures beneath the retinal pigment epithelium can be associated with serous PED, neurosensory detachment, subretinal hemorrhage, and lipid exudation, all of which are hallmarks of the disease (Fig. 1, 2).2
“PCV is a slow-growing, complex form of neovascularization that has a branching vascular network with aneurysmal dilations at the outer border of the network [Fig. 1],” Dr. Spaide said. The BVN usually expands over years, and the aneurysms can grow, disappear, or be replaced by new network vessels over time, he added.
These aneurysms appear to cause the more dramatic exudative manifestations of PCV.
“The presence of pigment epithelial detachments and subretinal hemorrhage—which are less common in AMD—are signs that there may be underlying PCV, especially when there is a lot of hemorrhage or large hemorrhagic PED,” said Adrian H. C. Koh, MBBS, FRCS, of Eye & Retina Surgeons in Singapore.
Epidemiology and Genetics
Unlike AMD, which is most common in older Caucasian women, PCV is seen more often in Asians, African-Americans, and men, according to Gregg T. Kokame, MD, clinical professor of surgery at the University of Hawaii John A. Burns School of Medicine in Honolulu. “While PCV may occur in younger patients, the majority of our Asian patients with PCV are in the same older age range as those with AMD,” Dr. Kokame added.
The comparison of genetic risk factors for PCV and AMD appears to be affected by race. Asian patients with typical CNV or AMD have different genetic markers for this disease than do Caucasian patients, Dr. Kokame said.
However, within an Asian population, the genetic markers for CNV and AMD are similar, he added. “This observation supports the concept that PCV is a type of subretinal neovascularization,” he said. Similarly, Dr. Spaide said, among Caucasians, PCV and AMD have similar genetic risk factors.
Given that the risk alleles are the same in PCV and AMD, their different disease expression suggests that there are genes or other factors that affect and modulate the clinical appearance of new vessel growth, according to Dr. Spaide. “Although these two diseases seem to be genetically similar, these findings may highlight the limitation of what we know about the genetics of neovascularization.”
Diagnosis: Choose ICG
Regardless of genetics, PCV has a distinctive appearance, particularly on indocyanine green (ICG) angiography, which is currently the best technique for differentiating it from other forms of neovascularization. According to Dr. Koh, ICG should be used when an ophthalmologist sees signs or symptoms that are suggestive of PCV, especially if they are unilateral and found in younger patients. Other red flags for the possible presence of PCV are lack of response to anti-VEGF agents in patients who have previously been diagnosed with AMD, massive submacular hemorrhage, and clinically apparent orange nodules beneath the retinal pigment epithelium, Dr. Koh said.
Even though ICG angiography is generally acknowledged as the standard for diagnosing PCV, it is not widely used for this purpose, except in Asian countries, where the prevalence of PCV is very high, according to Dr. Kokame.5 If ICG were used more routinely to diagnose PCV in white populations, he noted, its incidence among Caucasians might be higher than currently reported.
Using ICG to identify PCV is vital in guiding the choice and timing of treatment, said Dr. Kokame. For example, in some PCV patients, PDT may be a more effective therapy than the anti-VEGF agents used for AMD.
Treatment: PDT or Anti-VEGF?
Since 2002, research has demonstrated the effectiveness of PDT with verteporfin for PCV.6 Other clinical trials indicate that intravitreal injections of anti-VEGF agents can stabilize visual acuity and macular edema and achieve modest regression of PCV polyps. These therapeutic approaches are also being tested in combination, as, for example, in the EVEREST study.2
EVEREST. This multicenter, double-masked trial compared three treatment regimens—verteporfin PDT plus the anti-VEGF agent ranibizumab (Lucentis), ranibizumab monotherapy, and PDT monotherapy—in 61 Asian patients with symptomatic PCV. The primary end point was complete polyp regression as assessed by ICG. Patients were randomized to either verteporfin PDT plus three 0.5-mg intravitreal ranibizumab injections; verteporfin PDT plus three sham injections; or three 0.5-mg ranibizumab injections plus sham PDT. The PDT treatment and first ranibizumab or sham injection were delivered in one eye of each patient on the same day at baseline, and patients received two subsequent ranibizumab or sham injections between 3 and 5 months after baseline.2
The recently published six-month results revealed that PDT plus ranibizumab therapy and PDT monotherapy were both superior to ranibizumab monotherapy in achieving complete polyp regression (77.8 percent and 71.4 percent vs. 28.6 percent, respectively; p < 0.01). Patients in all three treatment arms had improvements in mean best-corrected visual acuity: 10.9 letters for verteporfin PDT plus ranibizumab, 7.5 for verteporfin PDT monotherapy, and 9.2 for ranibizumab monotherapy. However, the study was not powered to demonstrate statistically significant differences in BCVA outcomes. All treatments were well tolerated.
“We designed the EVEREST study to look at the angiographic outcomes because we wanted to see how successful the therapies were in achieving complete closure of polyps as seen on ICG, not just in controlling exudation and improving vision,” said Dr. Koh, who was one of the study investigators. “We found that the rate of closure of angiographic polyps was significantly increased in the PDT plus Lucentis arm and the PDT monotherapy arm compared with Lucentis alone.”
Although the visual acuity results of PDT plus anti-VEGF therapy may not be better than anti-VEGF alone, the advantages of using PDT include better regression of aneurysmal lesions and longer duration of effect in some patients, Dr. Spaide said. Its disadvantages include the potential complications of bleeding and exudative detachments, he added.
Comparative anti-VEGF studies. There have been some limited comparisons of different anti-VEGF therapies in PCV patients. A recent retrospective review of 121 patients with PCV found no significant differences between intravitreal bevacizumab and ranibizumab in BCVA outcomes or improvement in foveal center thickness. Patients received three or more injections of 1.25 mg intravitreal bevacizumab or 0.5 mg ranibizumab, and outcomes were assessed at 12 months.3 Polyp regression was also similar in the two treatment groups, with a regression rate of 24.2 percent in bevacizumab patients and 23.3 percent in the ranibizumab group.
Dr. Spaide said that there are currently no studies comparing aflibercept (Eylea) with ranibizumab or bevacizumab.
Perspectives on PCV management. In many cases, it may be appropriate to initiate treatment with anti-VEGF injection, said Dr. Kokame. In patients who respond to anti-VEGF, the potential but rare complications of PDT can be avoided—including subretinal or sub-RPE hemorrhages or choroidal nonperfusion, he wrote in a recent editorial in Retina.5
“If the patient’s vision is relatively good, and we are trying to get control of the leaking and bleeding, then we don’t go to PDT right away. But PDT is important to consider when anti-VEGF therapy is not effective or if vision worsens or is poor,” said Dr. Kokame. “In patients who do not respond to anti-VEGF therapy alone, we push to do PDT combined with intravitreal anti-VEGF and dexamethasone (Decadron) injection.” Typically, PDT is administered on the same day as anti-VEGF injection, although these therapies can also be administered a few days apart, Dr. Kokame noted.
Other considerations come into play, as well. For example, Dr. Kokame said, “We may also use PDT for patients who have to travel long distances for treatment because coming in for monthly injections of anti-VEGF therapy is more difficult for them.”
Whatever therapy is used, treatment of PCV tends to be less successful in patients with long-standing disease or in cases in which hemorrhage extends across the entire macula or even beyond it, Dr. Koh said. “These patients tend to end up with very nasty scars in the macula because of the destruction of photoreceptors. The result is poor visual outcomes.”
However, Dr. Spaide emphasized, patients with PCV can be treated successfully at length with current therapies. “We have patients who have had the disease for a long time and still have good vision. I take care of one patient who was involved in our first paper in 1990, and she still has 20/60 vision.”
___________________________
1 Laude A et al. Prog Retin Eye Res. 2010;29(1):19-29.
2 Koh A et al. Retina. 2012;32(8):1453-1464.
3 Cho HJ et al. Eye (Lond). 2012;26(3):426-433.
4 Yannuzzi LA et al. Retina. 1990;10(1):1-8.
5 Kokame GT. Retina. 2012;32(8):1446-1448.
6 Spaide RF et al. Retina. 2002;22(5):529-535.
___________________________
Dr. Koh receives honoraria from Allergan, Bayer, and Novartis. Dr. Kokame is a consultant to Alimera Sciences, Allergan, Genentech, and Santen Pharmaceuticals. Dr. Spaide reports no relevant financial disclosures.
American Academy of Ophthalmology Clinical Update: Retina December 2012


No comments yet.